-
Kitsap Respiratory Illness Report: Week 17 (4/20/2025 – 4/26/2025)
In week ending April 26, local indicators for influenza were low and continued to approach baseline levels, while indicators for COVID-19 and respiratory syncytial virus (RSV) remained minimal. Other Puget Sound counties observed similar trends. Regional laboratory surveillance most frequently detected rhinovirus among clinical respiratory specimens, followed by SARS-CoV-2 (COVID-19). Fewer than 10 emergency department (ED) visits were attributable to…
-
Health Advisory: Nirsevimab supplies limited
Advisory or Update, Communicable Disease and Immunization Update, Health Advisory, Provider Resources, VaccineNov. 22, 2023 Washington is scheduled to receive 7,700 50 mg and 600 100 mg Pfizer nirsevimab doses for the remainder of respiratory syncytial virus (RSV) season. The CDC released a Health Alert that provides guidance for healthcare providers. See the background section below for details. ACTIONS REQUESTED Immunize.org released a standing order template for this RSV season to ensure…
-
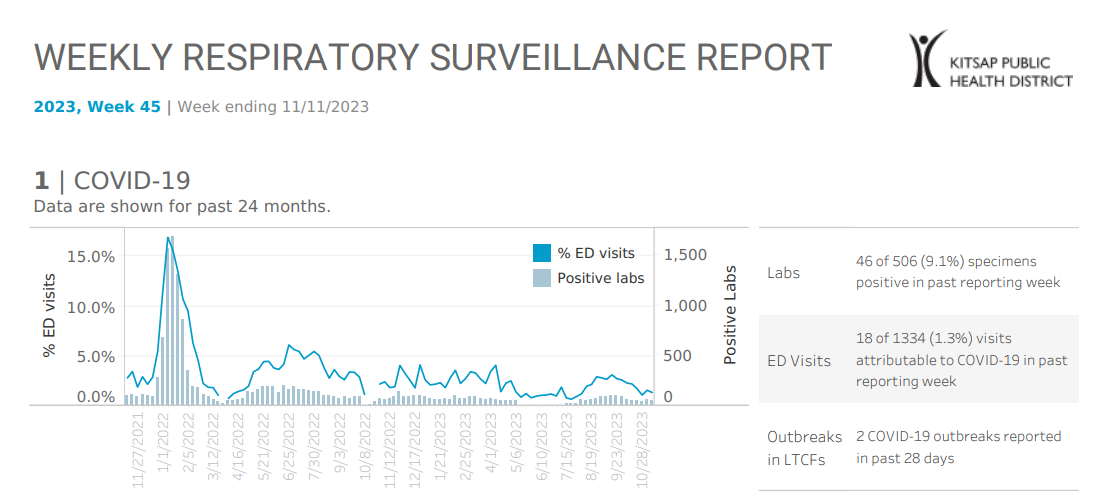
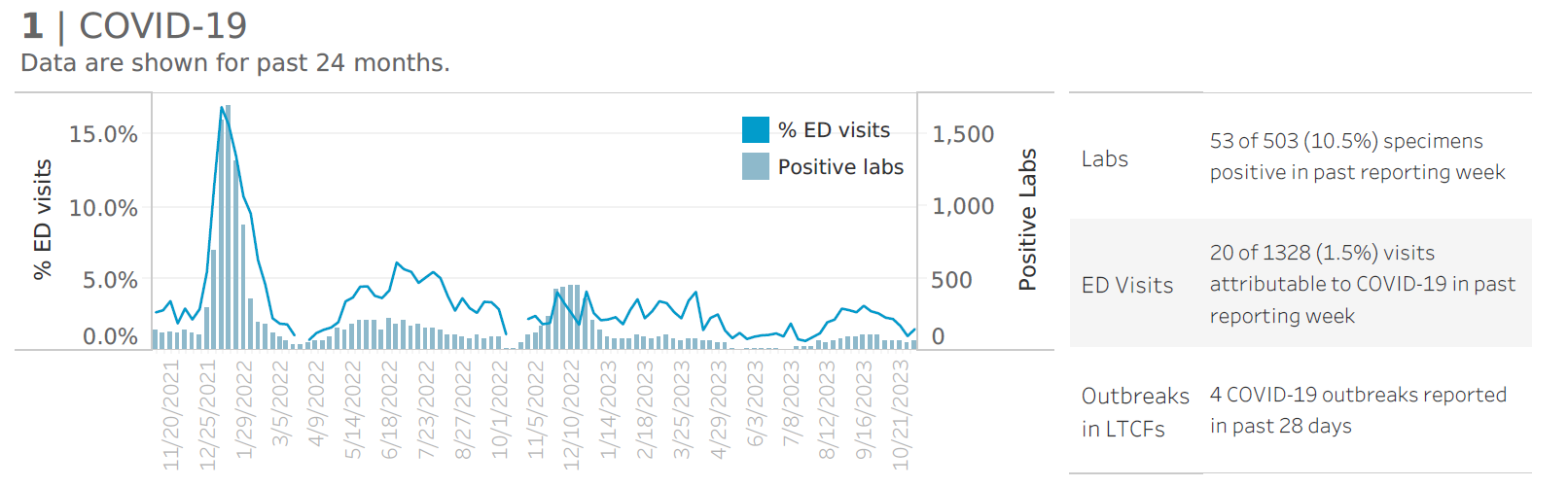
Kitsap Respiratory Illness Report: Nov. 5 to Nov. 11
Advisory or Update, Communicable Disease and Immunization Update, Communicable Disease Data Report, COVID-19, Health Advisory, Health Alert, Immunization, Immunizations, Influenza, Influenza Update, News and Alerts, News and Update, Notifiable Conditions, Respiratory Illness Report, Respiratory Illness ReportClick here to access week 45 of the Kitsap Respiratory Illness Report. In the week ending Nov. 11, Kitsap Public Health District saw increasing numbers of labs positive for influenza and respiratory syncytial virus (RSV) reported by our sentinel respiratory reporting laboratories, indicating that flu and RSV are rising locally. Local COVID-19 indicators remain low. 1.3%…
-
Kitsap Respiratory Illness Report: Oct. 29 to Nov. 4
Communicable Disease and Immunization Update, Communicable Disease Data Report, COVID-19, Health Advisory, Health Alert, Infection Control, Influenza, Influenza Update, News and Alerts, News and Update, Provider Resources, Respiratory Illness ReportClick here to access week 44 of the Kitsap Respiratory Illness Report. Local indicators for COVID-19, influenza and respiratory syncytial virus (RSV) remain low for week ending Nov. 4. 1.5% of emergency department visits were attributable to COVID-19 and there were fewer than ten emergency department (ED) visits attributable to either influenza or RSV. Kitsap sentinel…
-
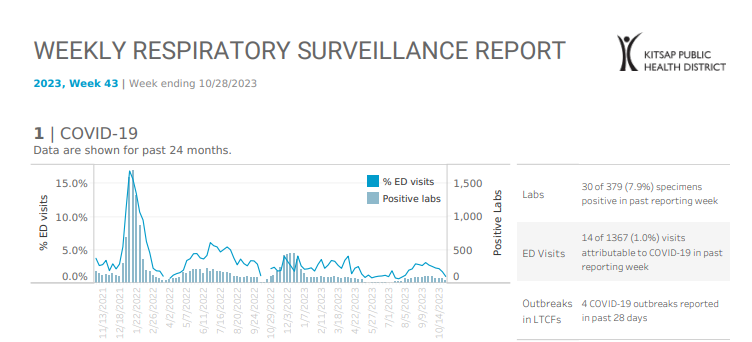
Kitsap Respiratory Illness Report: Oct. 22 to Oct. 28
Advisory or Update, Communicable Disease and Immunization Update, Communicable Disease Data Report, COVID-19, Health Advisory, Health Alert, Infection Control, Influenza, Influenza Update, News and Alerts, News and Update, Provider Resources, Respiratory Illness Report, Respiratory Illness ReportClick here to access week 43 of the Kitsap Respiratory Illness Report. Local indicators for COVID-19, influenza and respiratory syncytial virus (RSV) remain low for week ending 10/28/2023. 1.0% of emergency department visits were attributable to COVID-19 and there were fewer than ten emergency department (ED) visits attributable to either influenza or RSV. Kitsap sentinel reporting labs reported…
-
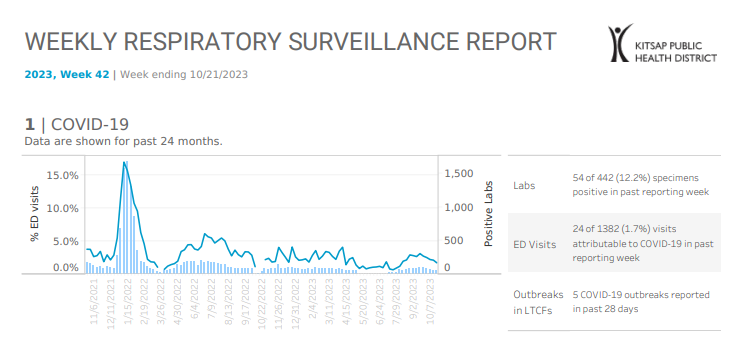
Kitsap Respiratory Illness Report: Oct. 15 to Oct. 21
Advisory or Update, Communicable Disease and Immunization Update, Communicable Disease Data Report, COVID-19, Health Advisory, Health Alert, Infection Control, Influenza, Influenza Update, News and Alerts, News and Update, Provider Resources, Respiratory Illness Report, Respiratory Illness Report, UncategorizedClick here to access week 42 of the Kitsap Respiratory Illness Report. Local indicators for COVID-19, influenza and respiratory syncytial virus (RSV) remain low for week ending 10/21/2023, although RSV activity appears to be increasing based on local lab data. 1.7% of emergency department visits were attributable to COVID-19 and none to influenza. Kitsap sentinel reporting…
-
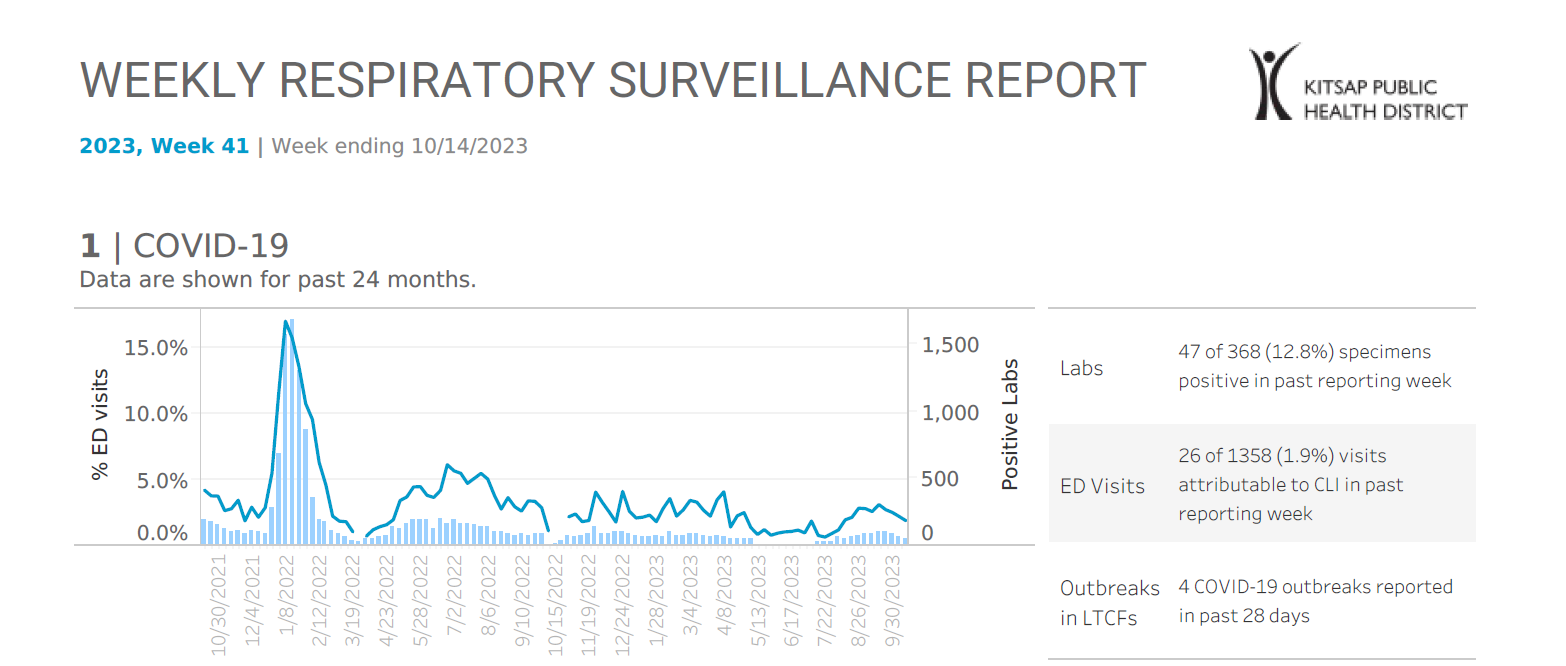
Kitsap Respiratory Illness Report: Oct. 8 to Oct. 14
Advisory or Update, Communicable Disease and Immunization Update, Communicable Disease Data Report, COVID-19, Health Advisory, Health Alert, Infection Control, Influenza, Influenza Update, News and Alerts, News and Update, Provider Resources, Respiratory Illness Report, Respiratory Illness ReportNote: We have refreshed our respiratory illness report format and will continue to make improvements. If you have questions or feedback regarding this report, please contact pio@kitsappublichealth.org. Click here to access week 41 of the Kitsap Respiratory Illness Report. Local indicators for COVID-19, influenza and respiratory syncytial virus (RSV) remain low for week ending 10/14/2023. 1.9% of emergency department visits…
-
Health Advisory: FDA authorized new Novavax COVID-19 vaccine formulation
Advisory or Update, Communicable Disease and Immunization Update, COVID-19, Health Advisory, Immunization, News and Update, Notifiable Conditions, Novel Coronavirus, Provider Resources, VaccineActions requested BE AWARE that on Oct. 3, 2023, U.S. Food and Drug Administration (FDA) authorized for emergency use a new adjuvanted (2023–2024) formulation of Novavax COVID-19 vaccine for people 12 years or older. BE AWARE that the FDA deauthorized the original Novavax COVID-19 vaccine. BE AWARE that the new adjuvanted (2023–2204) formulation of Novavax COVID-19 vaccine is…
-
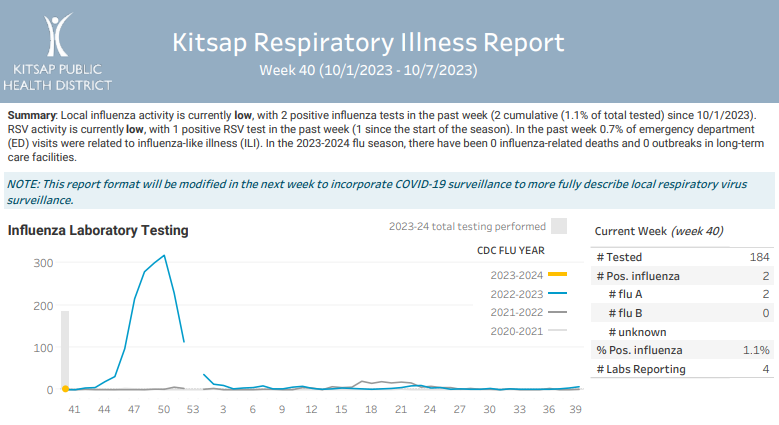
Kitsap Respiratory Illness Report: Oct. 1 to Oct. 7
Advisory or Update, Communicable Disease and Immunization Update, Communicable Disease Data Report, Health Advisory, Health Alert, Infection Control, Influenza, Influenza Update, Provider Resources, Respiratory Illness Report, Respiratory Illness ReportClick here to access week 40 of the Kitsap Respiratory Illness Report. Summary: Local influenza activity is currently low, with 2 positive influenza tests in the past week (2 cumulative (1.1% of total tested) since 10/1/2023). RSV activity is currently low, with 1 positive RSV test in the past week (1 since the start of the…
-
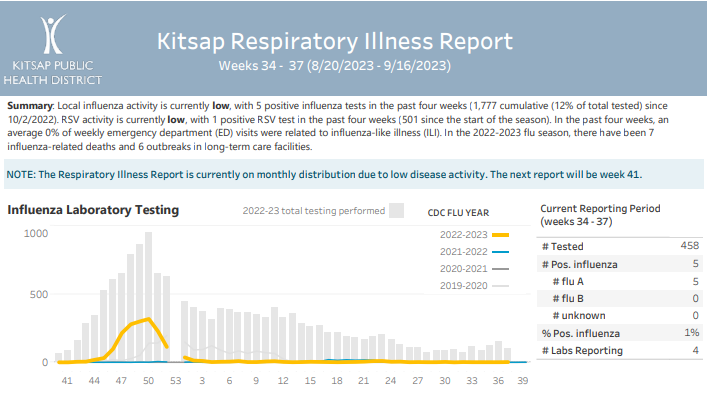
Kitsap Respiratory Illness Report: Aug. 20 to Sept. 16
Advisory or Update, Communicable Disease Data Report, Health Advisory, Health Alert, Influenza, Influenza Update, News and Alerts, News and Update, Provider Resources, Respiratory Illness Report, Respiratory Illness ReportClick here to access weeks 34 through 37 of the Kitsap Respiratory Illness Report. Summary: Local influenza activity is currently low, with 5 positive influenza tests in the past four weeks (1,777 cumulative (12% of total tested) since 10/2/2022). RSV activity is currently low, with 1 positive RSV test in the past four weeks (501 since…
-
Health Advisory: New COVID-19 vaccines authorized, previous bivalent COVID-19 vaccines deauthorized
Advisory or Update, Communicable Disease and Immunization Update, COVID-19, Health Advisory, Immunization, Immunizations, News and Alerts, News and Update, Provider Resources, VaccineActions requested BE AWARE that on Sept. 11, 2023, the U.S. Food and Drug Administration (FDA) approved and/or authorized for emergency use new, updated (2023–2024) COVID-19 vaccines from Moderna and Pfizer. Then, on Sept. 12, 2023, Centers for Disease Control and Prevention (CDC) recommended everyone 6 months or older get a new, updated (2023–2024) COVID-19 vaccine.…
-
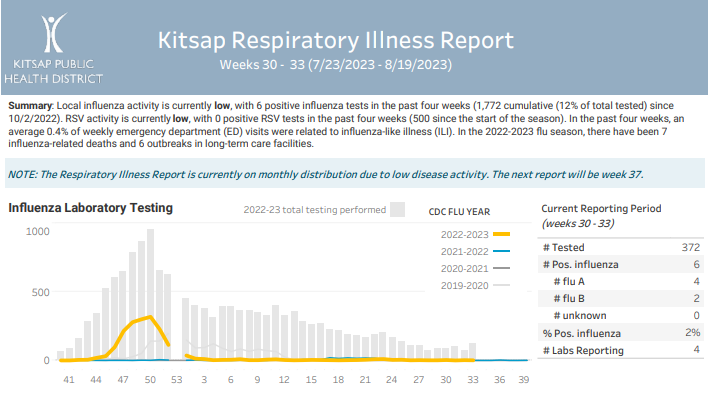
Kitsap Respiratory Illness Report: July 23-Aug. 19
Advisory or Update, Communicable Disease Data Report, Health Advisory, Health Alert, Infection Control, Influenza, Influenza Update, News and Alerts, News and Update, Provider Resources, Respiratory Illness Report, Respiratory Illness ReportClick here to access weeks 30 through 33 of the Kitsap Respiratory Illness Report. Summary: Local influenza activity is currently low, with 6 positive influenza tests in the past four weeks (1,772 cumulative (12% of total tested) since 10/2/2022). RSV activity is currently low, with 0 positive RSV tests in the past four weeks (500 since…
-
Health Advisory: Listeria contamination of restaurant milkshakes in Tacoma
Advisory or Update, Health Advisory, Health Alert, Infection Control, News and Update, Provider ResourcesActions requested Background The Washington State Department of Health announced that from Feb. 27 to July 22, 2023, six Washington residents (five from Pierce County and one from Thurston County) reported severe illness, which was linked to infection with Listeria bacteria (or listeriosis). All infected residents had weakened immune systems. Three residents died. Whole genome sequencing (or…