Kitsap Respiratory Illness Report: February 12 – February 18
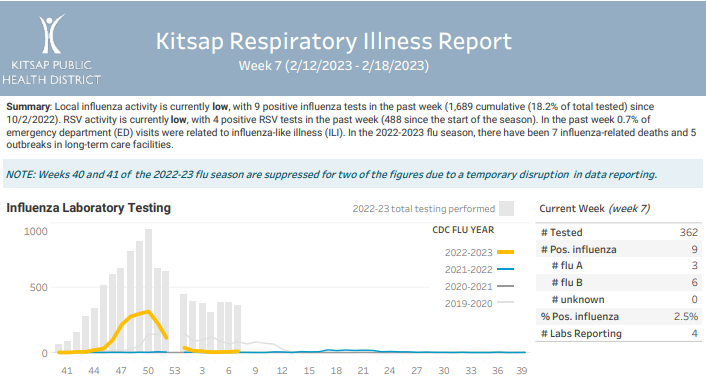
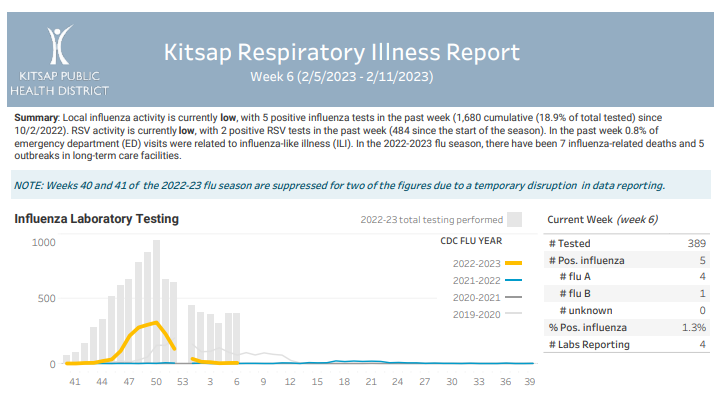
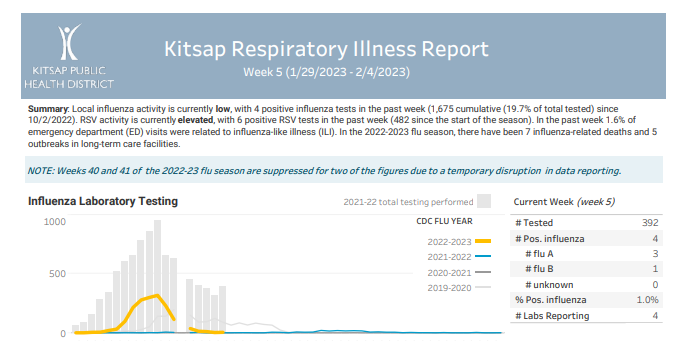
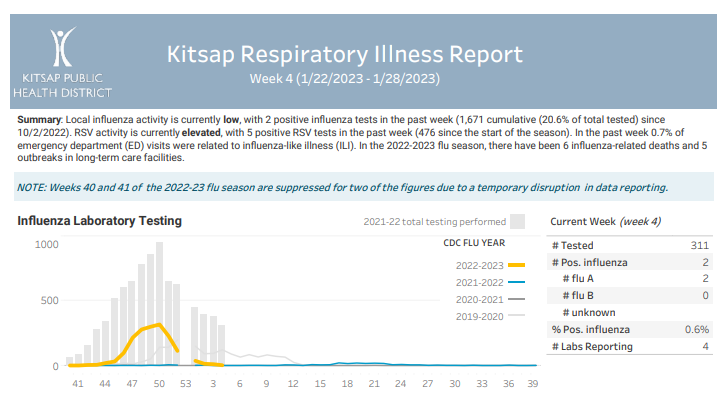
Click here to access week 7 of the Kitsap Respiratory Illness Report: 02.12.23-02.18.23. Summary: Local influenza activity is currently low, with 9 positive influenza tests in the past week (1,689 cumulative (18.2% of total tested) since 10/2/2022). RSV activity is currently low, with 4 positive RSV tests in the past week (488 since the start of … Kitsap Respiratory Illness Report: February 12 – February 18