-
Kitsap Respiratory Illness Report: June 23 – July 20
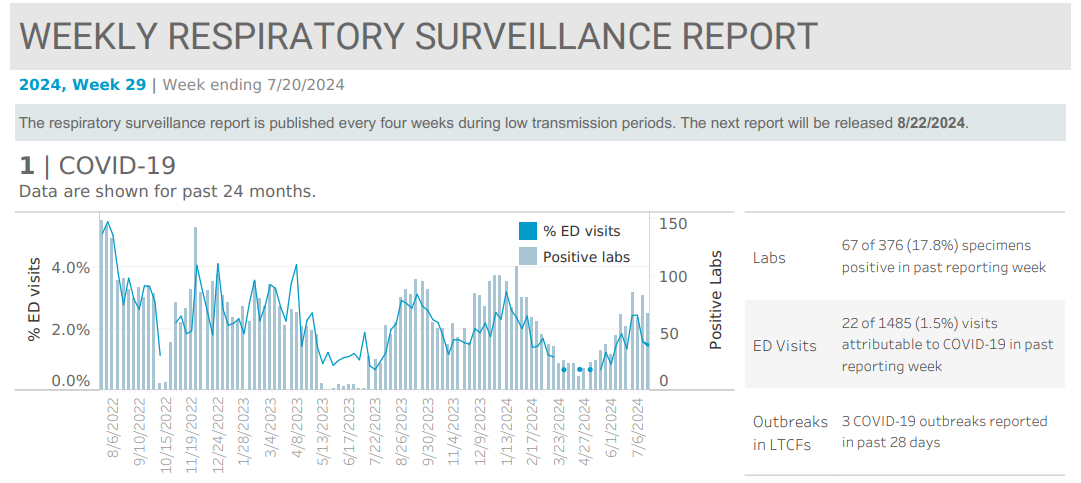
Communicable Disease and Immunization Update, Communicable Disease Data Report, COVID-19, Infection Control, Influenza, Influenza Update, News and Alerts, News and Update, Provider Resources, Respiratory Illness Report, Respiratory Illness ReportIn the week ending July 20, local indicators for COVID-19 were elevated, but appeared to be holding steady. Local indicators for influenza and respiratory syncytial virus (RSV) remained low. 1.5% of emergency department visits were attributable to COVID-19, and fewer than 10 emergency department (ED) visits were attributable to either influenza or RSV. Kitsap sentinel reporting…
-
Updated bivalent COVID-19 boosters authorized for children 6 months to 5 years old
Communicable Disease and Immunization Update, COVID-19, Immunization, Immunizations, News and Alerts, News and Update, Novel Coronavirus, Provider Resources, VaccineEarlier this month, U.S. Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) expanded emergency use authorization (EUA) of Moderna and Pfizer-BioNTech bivalent vaccines for children 6 months and older. Updated (bivalent) COVID-19 booster vaccines are formulated to boost immunity against the original coronavirus strain and protect against the newer omicron…
-
Newly Arrived Ukrainian Citizens’ Medical and Mental Health Needs
Behavioral Health, Immunization, Immunizations, News and Alerts, News and Update, Provider Resources, VaccineOn April 21, 2022, President Biden announced a new Uniting for Ukraine (U4U) process to help Ukrainian citizens fleeing war come to the United States temporarily. As of November 18, 2022: Newly arrived Ukrainian citizens have various medical and mental health needs. Resources
-
Kitsap Respiratory Illness Report: December 11 – December 17, 2022
Click here to access week 50 of the Kitsap Respiratory Illness Report: 12.11.2022 – 12.17.2022.
-
Kitsap Respiratory Illness Report: December 4 – December 10, 2022
Click here to access week 49 of the Kitsap Respiratory Illness Report: 12.04.2022 – 12.10.2022.
-
Kitsap Respiratory Illness Report: November 27 – December 3, 2022
Click here to access week 48 of the Kitsap Respiratory Illness Report: 11.27.2022 – 12.03.2022.
-
Guidance Issued for Managing Tamiflu Shortages
The state Department of Health has posted guidance for healthcare providers in response to Oseltamivir (Tamiflu) shortages being reported in some Washington communities. Healthcare providers and long-term care facilities that are treating influenza infections in high risk patients and are not able to obtain necessary supplies of Tamiflu should contact Kitsap Public Health District for…
-
Health Advisory: Respiratory Illness Resources
Advisory or Update, Communicable Disease and Immunization Update, Health Advisory, Health Alert, Infection Control, Influenza, News and Alerts, News and Update, Provider ResourcesActions Requested Sessions are labeled Session #1 and #2 – but both sessions have the same content and scope. Monday, December 12, 2022 8:30am-10:00am (PST) Session #1 for RNs, Techs, CNAs, and RTs: Microsoft Teams Meeting Click here to join the meeting Meeting ID: 240 163 698 56Passcode: qr3pni Join with a video conferencing device…
-
Kitsap Respiratory Illness Report: November 20 – November 26, 2022
Click here to access week 47 of the Kitsap Respiratory Illness Report: 11.20.2022 – 11.26.2022.
-
Kitsap Respiratory Illness Report: November 13 – November 19, 2022
Click here to access week 46 of the Kitsap Respiratory Illness Report: 11.13.2022 – 11.19.2022.
-
Health Advisory: Pediatric RSV Surge in Washington State
Advisory or Update, Communicable Disease and Immunization Update, Emerging Diseases and Conditions, Health Advisory, Health Alert, Immunization, Immunizations, Infection Control, News and Alerts, News and Update, Provider Resources, VaccineActions Requested Background WA Department of Health HAN 11/18/2022: The Centers for Disease Control and Prevention (CDC) issued a Health Alert Network (HAN) Health Advisory (11-04-2022 CDCHAN-00479) regarding an early surge in pediatric respiratory disease incidence caused by multiple viruses. With increased transmission and impacts on healthcare systems in Washington, this message is being sent…
-
Kitsap Respiratory Illness Report: November 6 – November 12, 2022
Click here to access week 45 of the Kitsap Respiratory Illness Report: 11.6.2022 – 11.12.2022.
-
Kitsap Respiratory Illness Report: October 30 – November 5, 2022
Click here to access week 44 of the Kitsap Respiratory Illness Report: 10.30.2022 – 11.5.2022.